Calcium fluoride’s high melting point is due to its strong ionic bonding between calcium and fluoride ions.
The electronegativity difference creates a powerful attraction, while its face-centered cubic crystal structure further enhances stability.
In addition, these factors demand substantial energy to break the bonds, resulting in a high melting point of about 1,418°C (2,584°F).
What Are the Main Factors Contributing to High Melting Point?
The elevated melting point of a substance is primarily influenced by its atomic structure, intermolecular forces, and the presence of various types of chemical bonding.
Ionic Bonding:
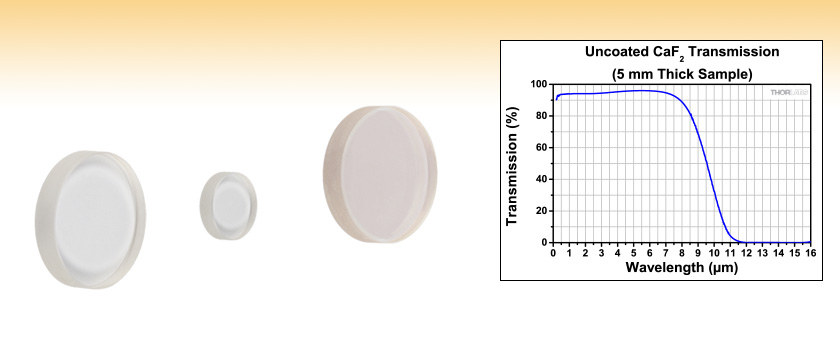
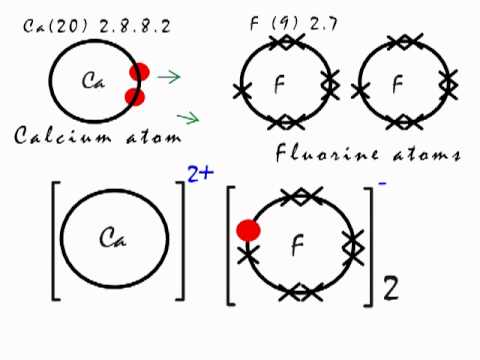
Calcium ions (Ca²⁺) and fluoride ions (F⁻): In calcium fluoride, each calcium atom loses two electrons to become a positively charged ion (Ca²⁺). And each fluorine atom gains one electron to become a negatively charged ion (F⁻).
Moreover, this electron transfer creates a strong electrostatic attraction between the oppositely charged ions.
The calcium ions are surrounded by a “shell” of negatively charged fluoride ions, and vice versa. This arrangement forms a stable ionic lattice structure.
Electronegativity difference: The electronegativity of an element indicates its ability to attract electrons in a chemical bond.
Moreover, calcium has a lower electronegativity, while fluorine has a higher electronegativity. This significant difference in electronegativity intensifies the attraction between calcium and fluoride ions, leading to a stronger ionic bond.
The resulting electrostatic forces between these ions are substantial and require a lot of energy to overcome, contributing to the high melting point.
Crystal Structure:
Face-centered cubic (FCC) lattice arrangement: In the solid state, calcium fluoride forms a face-centered cubic lattice, where calcium ions are positioned at the corners of each cube. And fluoride ions are situated at the center of each face of the cube.
Moreover, this arrangement results in a repeating pattern that maximizes the distance between ions of the same charge while minimizing the distance between ions of opposite charge. This spatial arrangement enhances the overall stability of the crystal lattice.
Maximizing attraction between ions: The face-centered cubic arrangement optimizes the attraction between the positively charged calcium ions and the negatively charged fluoride ions.
Additionally, the ions are positioned in a way that allows them to be as close as possible while maintaining the alternating pattern of positive and negative charges.
Moreover, this proximity further strengthens the electrostatic forces between the ions, making it more difficult to break the bonds and transition the substance to a liquid state.
How Can It Effects on Energy Requirements?
The impact of high melting points on energy requirements is profound, as substances with elevated melting points often necessitate higher amounts of energy.
Energy needed to break ionic bonds:
Ionic bonds are formed when electrons are transferred from one atom to another, resulting in positively and negatively charged ions that attract each other due to their opposite charges.
Moreover, breaking these ionic bonds requires energy to overcome the electrostatic forces holding the ions together.
In the case of calcium fluoride (CaF2), the ionic bonds between the calcium ions (Ca²⁺) and fluoride ions (F⁻) are exceptionally strong due to the large electronegativity difference between calcium and fluorine.
Deeper Insight:
The energy required to break ionic bonds is related to the magnitude of the charges on the ions and the distance between them.
In calcium fluoride, the charges on the ions are relatively high (Ca²⁺ and F⁻) and they are held close together in the crystal lattice.
As a result, a significant amount of energy is needed to overcome the strong attraction between these oppositely charged ions.
Transition from solid to liquid state:
When a substance transitions from a solid to a liquid state, it undergoes a phase change where the arrangement of particles changes from a highly ordered structure in the solid to a more disordered state in the liquid.
In addition, this phase transition requires the input of energy to break the intermolecular forces that hold the solid together.
Deeper Insight:
In the case of calcium fluoride, transitioning from the solid state (crystalline lattice) to the liquid state involves overcoming not only the strong ionic bonds between the calcium and fluoride ions but also the lattice structure itself.
As heat is applied, the energy breaks the ionic bonds, allowing the ions to move more freely. However, before the substance completely liquefies, the lattice structure needs to be disrupted, and this requires additional energy.
What Is The High Melting Point of Calcium Fluoride?
It’s robust ionic bonding and crystal lattice structure, making it valuable for various industrial applications.
Magnitude of melting point:
The melting point of a substance is the temperature at which it transitions from a solid to a liquid state. In the case of calcium fluoride (CaF2), the melting point is particularly high, at around 1,418°C (2,584°F).
Furthermore, this means that at temperatures below the melting point, calcium fluoride exists as a solid, and it requires a substantial amount of heat energy to transform it into a liquid.
Relation to ionic bonding and crystal structure:
The high melting point of calcium fluoride is closely tied to its strong ionic bonding and its crystalline lattice structure.
Ionic Bonding: As mentioned earlier, calcium fluoride is composed of calcium ions (Ca²⁺) and fluoride ions (F⁻) held together by ionic bonds.
Moreover, ionic bonds are among the strongest types of chemical bonds due to the electrostatic attraction between the oppositely charged ions. The energy needed to break these bonds and separate the ions is directly related to the strength of this attraction.
In addition, calcium fluoride’s significant electronegativity difference between calcium and fluorine amplifies this attraction, leading to robust ionic bonding. As temperature rises towards the melting point, energy is supplied to the substance, causing the ions to vibrate more vigorously. However, breaking the ionic bonds and overcoming the electrostatic forces between the ions requires a substantial input of energy.
Crystal Structure: The face-centered cubic (FCC) lattice arrangement in calcium fluoride further contributes to its high melting point.
In the solid state, the ions are arranged in a repeating pattern that maximizes the attraction between oppositely charged ions while minimizing the repulsion between ions of the same charge. This arrangement results in a highly ordered and stable structure.
As temperature increases, the lattice vibrations become more energetic. But the organized nature of the lattice structure makes it challenging for the ions to move past one another and transition into a liquid state.
What Are Real-world Applications?
Calcium fluoride’s unique properties, such as its high melting point and strong ionic bonding, make it valuable for various applications across different fields.
Optics and Photonics: Calcium fluoride is known for its excellent optical properties, including high transparency in the ultraviolet (UV) and infrared (IR) regions of the electromagnetic spectrum.
It is often used as a substrate material for optical components, such as lenses, windows, and prisms, in UV and IR applications.
Fluoride Flux: In metallurgical processes, calcium fluoride serves as a flux to lower the melting point of certain metal oxides and facilitate their removal during refining.
This property is exploited in the production of metals like aluminum and uranium.
Aluminum Industry: The aluminum industry utilizes calcium fluoride as a flux in the electrolytic reduction of alumina (aluminum oxide).
Moreover, it helps lower the melting point of the alumina and enhances the efficiency of the aluminum production process.
Ceramics and Glass: Calcium fluoride’s high melting point and thermal stability make it suitable for use as a refractory material in ceramics and glass manufacturing.
Additionally, it can withstand high temperatures and harsh conditions without undergoing significant degradation.
Industrial and Scientific Applications:
Calcium fluoride’s properties find application in various industries and scientific endeavors.
Spectroscopy: The optical transparency of calcium fluoride in the UV and IR regions is particularly valuable in spectroscopy, where it is used as a window material for spectroscopic analysis of samples. Its low absorption in these regions allows accurate measurements.
Laser Technology: Calcium fluoride is employed as a laser gain medium for certain types of lasers, including excimer lasers. Moreover, its high thermal conductivity and optical quality are advantageous for generating and amplifying laser light.
Fluoride Ion Selective Electrodes: In analytical chemistry, calcium fluoride is used to create fluoride ion selective electrodes for measuring fluoride ion concentrations in solutions.
Dental and Bone Health: Calcium fluoride has been used in dental products like toothpaste and dental treatments due to its potential benefits for strengthening tooth enamel and preventing tooth decay.
Additionally, fluoride compounds are used to promote bone health and prevent osteoporosis.
Pharmaceuticals: Calcium fluoride nanoparticles are being explored for drug delivery systems due to their biocompatibility and ability to encapsulate and release therapeutic agents.
FAQ’s
Why does calcium have a high melting point?
Calcium’s high melting point is due to its strong metallic bonding resulting from the delocalization of electrons across its lattice structure.
Why does calcium oxide have a higher melting point than potassium fluoride?
Calcium oxide’s higher melting point is attributed to its stronger ionic bonding resulting from higher charges on calcium ions and oxygen ions compared to potassium fluoride’s ions.
Why does potassium fluoride have a high melting and boiling point?
Potassium fluoride’s high melting and boiling points stem from its strong ionic bonding between potassium and fluoride ions, requiring substantial energy to break.
Why does potassium fluoride have a high melting point simply?
Potassium fluoride’s high melting point is due to its strong ionic bonds, resulting from the electrostatic attraction between positively charged potassium ions and negatively charged fluoride ions.
Why do substances have high melting points?
Substances have high melting points due to strong intermolecular forces, such as covalent, metallic, or ionic bonds, that require significant energy input to break and transition the substance to a liquid state.
Why is the melting point so high?
A high melting point is a result of strong bonding forces, whether covalent, ionic, or metallic, which necessitate substantial energy to overcome and facilitate the transition from solid to liquid.
Final Thought
In conclusion, the high melting point of calcium fluoride is a result of its unique combination of strong ionic bonding and organized crystal lattice structure.
Moreover, the electronegativity difference between calcium and fluoride ions leads to powerful electrostatic attractions, forming robust ionic bonds that demand significant energy to break.
The face-centered cubic lattice arrangement further enhances stability, making it difficult for the crystal structure to transition from solid to liquid.
As a consequence, calcium fluoride exhibits a high melting point of around 1,418°C (2,584°F). This property finds practical applications in diverse fields, including optics, metallurgy, spectroscopy, ceramics, and dental care.
Finally, understanding the relationship between its properties and its high melting point is essential for harnessing the potential of calcium fluoride in various industrial and scientific contexts.