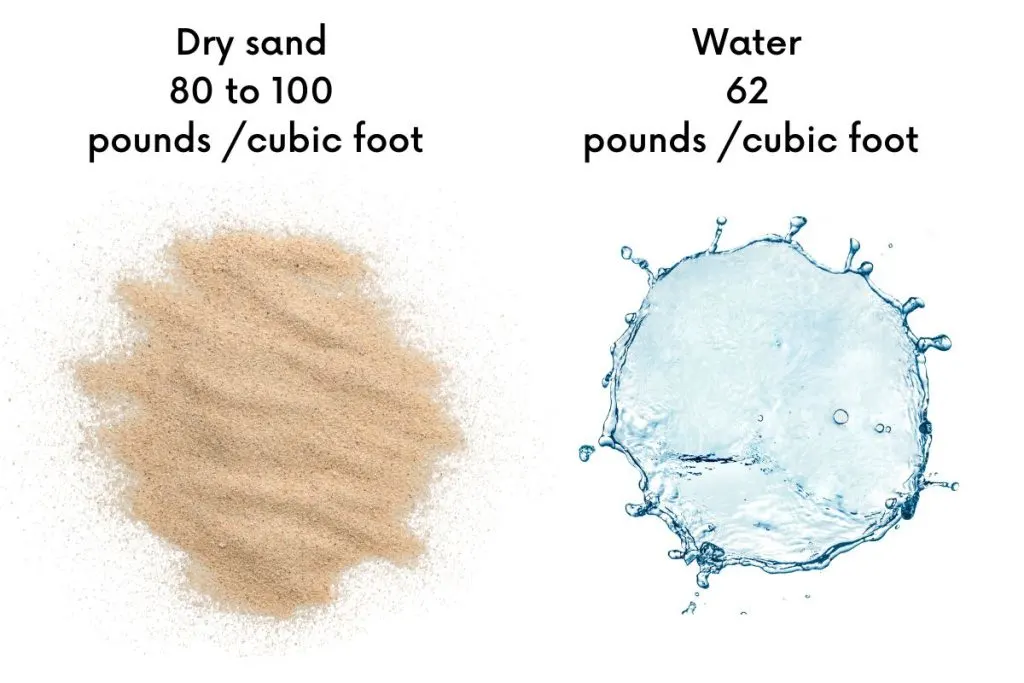
Sand, it carries more weight than water due to its compactness. In a small area like a shoebox, dry sand can be much heavier, ranging from 80 to 100 pounds, depending on its type.
Contrastingly, water feels lighter in that same space, weighing about 62 pounds. To make it easier, think of a liter of dry sand as resembling 1.5 small bags of sugar in weight, while a liter of water feels like carrying only 1 bag of sugar.
Discovering the variance in weight between these two common liquids can be quite beneficial for your house, garden, and the aquatic life that resides there. Making better decisions when it comes to structural design, landscaping, and other matters can be aided by understanding the difference in weight between water and sand.
Learn more about the weight disparity between sand and water and how it may affect your life by reading on.
Why does some sand float in water?

When sand and water are combined, you might have observed that the sand gradually settles at the container’s bottom. This occurrence aligns with our understanding that sand’s weight surpasses that of water, causing it to sink.
However, you may have also noticed that the introduction of sand sometimes imparts a cloudy appearance to the water, and particles of sand might seem to linger on or within the water.
In general, dry sand weighs around 1.5 kilograms per liter, whereas water registers at about 1 kilogram per 1 liter.
The rationale behind this phenomenon lies in the nature of sand itself. Sand isn’t just sand; it can harbor various components, including remnants of plants or soil, some of which are minuscule and lightweight.
Upon sand’s amalgamation with water, these delicate elements tend to float.
Furthermore, as individual grains of sand interact, they can break down into fine particles – giving rise to dust that holds minimal weight.
This dust often contributes to the cloudiness that arises when sand and water intermingle.
Given enough time without any disruption, this dust gradually becomes saturated with water and eventually descends to the container’s base. This, in turn, renders the water above relatively transparent.
Why do sand and water weigh differently under different conditions?”
The estimation of the weight of substances like sand and water is a common practice, yet it’s important to recognize that their exact weights can exhibit variability due to a range of factors. Take, for instance, sand, which can encompass a spectrum of grain sizes, leading to fluctuations in its weight. This variability in coarseness is intricately linked to its overall weight, along with the potential presence of diverse materials like rock fragments or shell pieces.
Similarly, the weight of water is intricately tied to its composition. While we acknowledge that saltwater holds greater weight than freshwater due to its dissolved salt content, it’s noteworthy that even within freshwater, the presence of dissolved particles like minerals can contribute to variations in weight.
Beyond composition, the temperature of water emerges as a notable influence. The expansion of warm or hot water brings about a slight decrease in weight due to its increased volume, making it occupy more space within a given container.
Contrarily, the contraction of cold water can lead to a marginal increase in weight as it occupies a slightly smaller volume than its room temperature counterpart.
Moreover, understanding the weight of these substances involves grasping fundamental concepts:
- Mass: The mass of an object signifies its matter content, and a higher mass invariably leads to a greater weight. Essentially, a larger number of particles or molecules contribute to the overall weight of the object.
- Acceleration Due to Gravity: The unchanging constant of gravity’s force on an object is pivotal. This force, approximately 9.81 meters per second squared on Earth’s surface, determines an object’s weight. The relationship between an object’s mass and the acceleration due to gravity is captured by the formula: Weight = Mass × Acceleration Due to Gravity.
Looking specifically at sand and water:
- Sand: Constituting solid particles with varying densities and sizes, sand’s weight per unit volume hinges on its density. This density is shaped by factors encompassing the composition of its particles, such as minerals or quartz, and the interstitial air spaces between these particles.
- Water: The weight of water is underpinned by its density and volume. At room temperature, pure water boasts a density around 1000 kilograms per cubic meter (kg/m³). It’s worth noting that this density can undergo subtle modifications due to temperature variations and changes in pressure.
Is wet sand heavier than dry sand?

While water boasts lower density than sand, one might assume that introducing water to sand would result in sand becoming lighter compared to an equivalent volume of dry sand.
However, this notion requires clarification. Dry sand is characterized by gaps between its individual particles, which are filled with air. Conversely, wet sand is permeated by water between these particles, contributing to enhanced density. As a consequence, wet sand exhibits greater mass, making it heavier than its dry counterpart.
It’s worth noting that if water content surpasses sand content, the scenario changes. In this instance, wet sand containing more water than sand would indeed weigh less than dry sand.
Calculating the Weight Difference between Water and Sand
Analyzing the weight contrast between water and sand unveils essential insights, shaping our understanding of how each substance influences diverse contexts. A practical method to establish their weight discrepancy involves measuring out a gallon of each material and calculating the ensuing pound differential. This straightforward approach delivers a direct comparison of their individual weights.
Another insightful avenue involves juxtaposing the weight of a cubic foot of both substances. This perspective intricately illustrates how the volume of each material interplays with your project or design considerations.
Further, Irrespective of your chosen methodology, it’s imperative to internalize that water outpaces sand by approximately 8.3 pounds per gallon in weight.
This revelation bears tangible repercussions.
In addion, When devising architectural constructs, the choice between water and sand necessitates meticulous evaluation, given that water’s weight mandates augmented structural reinforcement.
Conversely, if your creative endeavors encompass landscape compositions featuring sand, a larger volume becomes imperative compared to water. A profound grasp of this weight differential between the two substances empowers you to craft designs that harmonize both safety and resource optimization.
Impact on Aquatic Life
Unlocking the knowledge of the weight divergence between water and sand opens a gateway to safeguarding underwater ecosystems. The substantial difference in density between these elements can set off a chain reaction impacting aquatic life.
Additionally, introducing sand to bodies of water, like lakes and ponds, triggers a cautionary tale – its denser nature leads to settling, potentially choking aquatic flora, intensifying sediment buildup, and impinging on the essential oxygen supply vital for fish and other underwater inhabitants.
This narrative delves deeper; the presence of sand particles can disrupt fish respiration by clogging their gills, disrupting the delicate equilibrium.
Championing the well-being of aquatic life mandates an appreciation of the weight dissonance between water and sand.
Further, this understanding proves pivotal to preempt any unintended repercussions stemming from the infusion of sand into aquatic habitats. Thoughtful restraint takes the forefront when integrating sand, advocating for measured additions and systematic spreading to mitigate adverse sedimentation repercussions.
Vigilant guardianship of the impacted aquatic spaces emerges as a cornerstone. Such watchful stewardship assures the ongoing vitality of the submerged ecosystem.
Moreover, Equally pivotal is the selection of sand with care, ensuring its freedom from pollutants or contaminants detrimental to aquatic inhabitants. By internalizing the intricacies of the weight variance between water and sand, and translating this comprehension into mindful actions, we etch a narrative that champions the conservation and flourishing of aquatic life.
FAQs
What’s heavier than sand?
Various substances can be heavier than sand, depending on their densities and compositions. Some examples of substances that are generally heavier than sand include metals like lead, gold, and platinum, as well as some minerals and compounds with high densities.
How heavy is water?
Water boasts a density of roughly 1 gram per cubic centimeter (g/cm³), equivalent to 1 kilogram per liter (kg/L). This implies that a volume of 1 liter of water corresponds to a weight of 1 kilogram or 1000 grams.
Is a bucket of sand heavier than a bucket of water?
Yes, In terms of volume, beach sand outweighs water due to its higher density. The density of sand is about 1.5 times that of water, which means that a bucket filled with beach sand will be heavier than an equivalent bucket filled with water. This difference in density underlines the weight variation between these common substances
Conclusion
In the realm of weight comparison, the distinction between water and sand becomes a fascinating exploration. While sand emerges as the denser contender, its weightiness lends both benefits and considerations in various applications. Understanding the weight differential between water and sand holds practical significance, influencing choices from structural design to environmental preservation.